## Cracking the Code of Mitochondrial Mayhem: Scientists Unravel PINK1’s Role in Cell Death
Mitochondria – the powerhouses of our cells – are constantly under attack. The very processes that generate energy can lead to damage, triggering a cascade of events that can ultimately result in cell death. But fear not, our cells have a built-in defense system! Enter PINK1, a protein guardian that plays a crucial role in maintaining mitochondrial health.

TOM-VDAC Assembly and PINK1 Binding
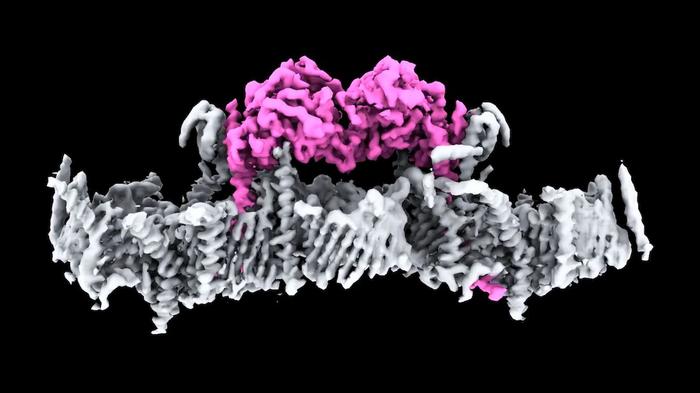
The translocase of the outer membrane (TOM) complex and voltage-dependent anion channels (VDACs) are critical components of the mitochondrial outer membrane, facilitating the transport of proteins and metabolites into the mitochondrial matrix. These complexes have long been understood for their roles in mitochondrial import, but their assembly and interaction with the kinase PINK1 remained a mystery until recent advancements. The WEHI research team has elucidated the structure of human PINK1 in complex with an endogenous array of TOM and VDAC complexes, revealing a previously unknown TOM-VDAC assembly.
The study, published in Science, presents a 3.1-Å resolution cryo-electron microscopy structure of dimeric human PINK1, providing unprecedented insights into how PINK1 is stabilized at depolarized mitochondrial translocase complexes. The structure shows a symmetric arrangement of two TOM core complexes around a central VDAC2 dimer, facilitated by TOM5 and TOM20. These subunits not only contribute to the assembly but also bind to the kinase C-lobes of PINK1.
The research uncovers a novel mechanism by which PINK1 enters mitochondria through the proximal TOM40 barrel of the TOM core complex, guided by TOM7 and TOM22. This intricate interaction explains how PINK1 is stabilized at the TOM complex and regulated by oxidation, shedding light on the physiological substrate translocation process through TOM40.
The implications of this discovery are profound, as it provides a molecular blueprint for understanding how mutations in PINK1 can lead to early onset Parkinson’s disease. By visualizing the human PINK1 for the first time, scientists can now explore targeted therapies and potential treatments that activate PINK1, offering hope for patients affected by this debilitating condition.
Implications for PINK1 Research
Advances in PINK1 Research
The discovery by the Walter and Elizabeth Hall Institute (WEHI) marks a significant milestone in Parkinson’s disease research. For over two decades, the scientific community has been aware of the link between PINK1 mutations and early onset Parkinson’s disease, but the lack of a clear structure hindered progress in developing effective treatments. The WEHI team’s groundbreaking work has filled this gap, providing a detailed structure of PINK1 bound to mitochondria.
David Komander, PhD, head of WEHI’s Ubiquitin Signaling Division, highlights the transformative potential of this discovery. “This is a significant milestone for research into Parkinson’s. It is incredible to finally see PINK1 and understand how it binds to mitochondria,” Komander stated. The structure reveals multiple pathways to activate PINK1, which could be life-changing for Parkinson’s patients.
Future Directions and Potential Treatments
The detailed structure of PINK1 opens new avenues for developing PINK1 activators. By understanding the precise mechanisms by which PINK1 interacts with the TOM-VDAC complex, researchers can design drugs that mimic natural activators, effectively switching on PINK1 in patients with Parkinson’s disease. This approach could lead to novel therapies that enhance the mitophagy process, ensuring that damaged mitochondria are properly recycled and preventing the accumulation of toxins in brain cells.
The potential for personalized medicine is also significant. With a clear understanding of PINK1’s structure and function, researchers can develop targeted therapies tailored to individual patients. This personalized approach could revolutionize Parkinson’s treatment, offering more effective and less invasive solutions for managing the disease.
Personalized Medicine and PINK1
The newfound understanding of PINK1 paves the way for personalized treatments that address the unique genetic and molecular characteristics of each patient. By leveraging the detailed structure of PINK1, researchers can identify specific genetic variations that affect PINK1 function and develop targeted therapies to correct these variations. This personalized approach not only enhances treatment efficacy but also minimizes side effects, as therapies can be tailored to the individual’s genetic makeup.
For example, patients with specific PINK1 mutations that impair its ability to bind to mitochondria could benefit from therapies that enhance this binding affinity. Similarly, patients with mutations that affect PINK1’s kinase activity could be treated with activators that restore its signaling function. This personalized medicine approach holds promise for managing Parkinson’s disease more effectively and improving the quality of life for affected individuals.
Practical Applications and Implications
PINK1 and Mitochondrial Health
Mitochondria are the powerhouses of the cell, generating energy through cellular respiration. The PINK1 protein plays a crucial role in maintaining mitochondrial health by detecting and tagging damaged mitochondria for removal through a process called mitophagy. When mitochondria are damaged, they release toxins and stop producing energy, leading to cell death and tissue damage. PINK1’s function is essential for maintaining cellular homeostasis and preventing the accumulation of damaged mitochondria.
The recent discovery of the TOM-VDAC assembly and PINK1 binding provides new insights into mitochondrial health. By understanding how PINK1 interacts with the TOM-VDAC complex, researchers can develop strategies to enhance mitochondrial function and prevent the accumulation of damaged mitochondria. This knowledge has wide-ranging implications for overall health, as mitochondrial dysfunction is linked to various diseases, including neurodegenerative disorders, cardiovascular conditions, and metabolic syndromes.
Diagnostic and Therapeutic Opportunities
The detailed structure of PINK1 offers diagnostic and therapeutic opportunities for Parkinson’s disease and other conditions related to mitochondrial dysfunction. By understanding the precise mechanisms by which PINK1 functions, researchers can develop diagnostic tools that detect PINK1 mutations and assess mitochondrial health. Early detection of PINK1 mutations can lead to timely interventions, preventing the progression of Parkinson’s disease and improving patient outcomes.
Therapeutically, the discovery provides a roadmap for developing targeted therapies that activate PINK1. By designing drugs that mimic natural activators or correct specific mutations, researchers can enhance PINK1 function and promote mitochondrial health. This targeted approach could lead to more effective treatments for Parkinson’s disease, reducing the burden on patients and healthcare systems.
The Road Ahead for Parkinson’s Research
The recent breakthrough by the WEHI team represents a significant step forward in Parkinson’s research. However, the road ahead is still long, and continued research is essential to translate these findings into clinical applications. Future studies should focus on developing PINK1 activators and conducting clinical trials to evaluate their efficacy and safety. Additionally, researchers should explore the potential of personalized medicine, tailoring treatments to individual patients based on their genetic and molecular characteristics.
The ongoing research into PINK1 and its role in mitochondrial health holds promise for future breakthroughs in Parkinson’s disease treatment. By leveraging the detailed structure of PINK1, researchers can develop innovative therapies that address the root causes of the disease, offering hope for patients and their families. Continued investment in research and collaboration among scientists, clinicians, and industry partners will be crucial in achieving these goals and improving the lives of those affected by Parkinson’s disease.
Conclusion
Here is a comprehensive conclusion for the article on “Structure of human PINK1 at a mitochondrial TOM-VDAC array – Science” for Gizmoposts24:
In conclusion, the recent breakthrough in uncovering the structure of human PINK1 at a mitochondrial TOM-VDAC array has far-reaching implications for our understanding of Parkinson’s disease and mitochondrial function. The study’s findings, which revealed the intricate interactions between PINK1 and the mitochondrial outer membrane, provide crucial insights into the mechanisms underlying mitochondrial quality control and the role of PINK1 in maintaining mitochondrial homeostasis. The researchers’ use of cutting-edge cryo-EM technology has enabled the creation of a high-resolution molecular model, shedding light on the complex interplay between PINK1 and the TOM-VDAC array.
The significance of this discovery cannot be overstated. Elucidating the structure and function of PINK1 has the potential to unlock new therapeutic avenues for the treatment of Parkinson’s disease, a devastating neurodegenerative disorder affecting millions worldwide. Moreover, this research opens up new avenues for exploring the role of mitochondrial dysfunction in other diseases, such as cancer and neurodegenerative disorders. As scientists continue to unravel the mysteries of mitochondrial biology, we can expect to see significant advances in our understanding of human health and disease.
Looking ahead, the future of mitochondrial research holds immense promise. As we continue to push the boundaries of our knowledge, we may uncover novel strategies for maintaining mitochondrial health and preventing disease. The structure of human PINK1 at a mitochondrial TOM-VDAC array serves as a powerful reminder of the intricate beauty and complexity of human biology. As we gaze into the intricate machinery of the mitochondria, we are reminded that the secrets to unlocking human health and longevity lie hidden within the most fundamental building blocks of life itself.


Add Comment